Project background & challenge
Since 2017, Becton, Dickinson and Company (“BD”) has been implementing a transformation of its production network for manufacturing glass syringes in various production sites around the world, in compliance with quality, customer and regulatory requirements.
The production of medical device components such as glass syringes obeys very strict regulatory standards such as ISO 13485 and specific specifications from pharmaceutical customers, implying a large number of constraints during implementation. With the “Beyond” program, BD is transforming its production network to strengthen its capacity to produce where it makes the most sense in terms of logistics flow.
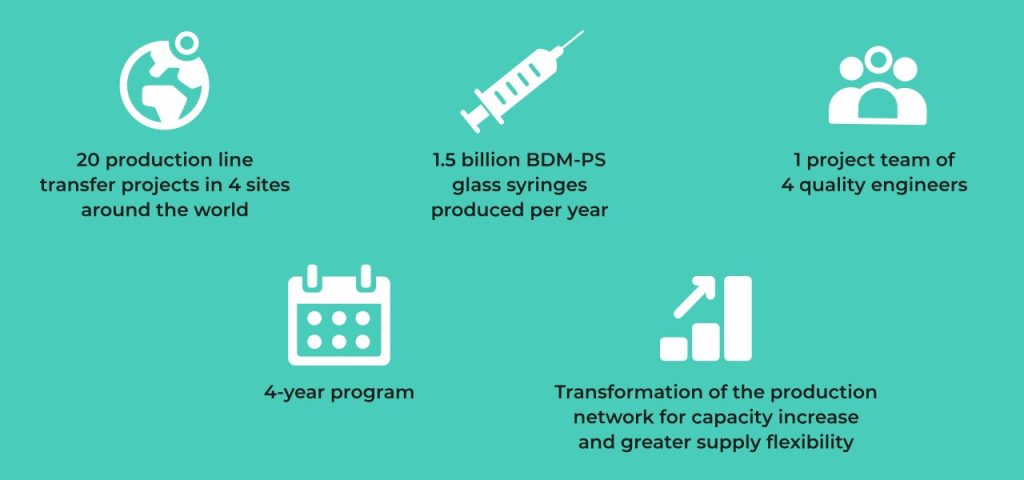
Solution
In order to ensure a transfer of production lines without impact on quality or market disruption, Expleo played a key role in transfer projects, from the initial preparation to production roll-out.
Expleo contributed to the transfer plan, providing technical assistance, quality management and factory support until the transfer was validated. Expleo has of course accompanied each of the key stages of the projects with quality documentation, proof of the successful completion of these transfers.
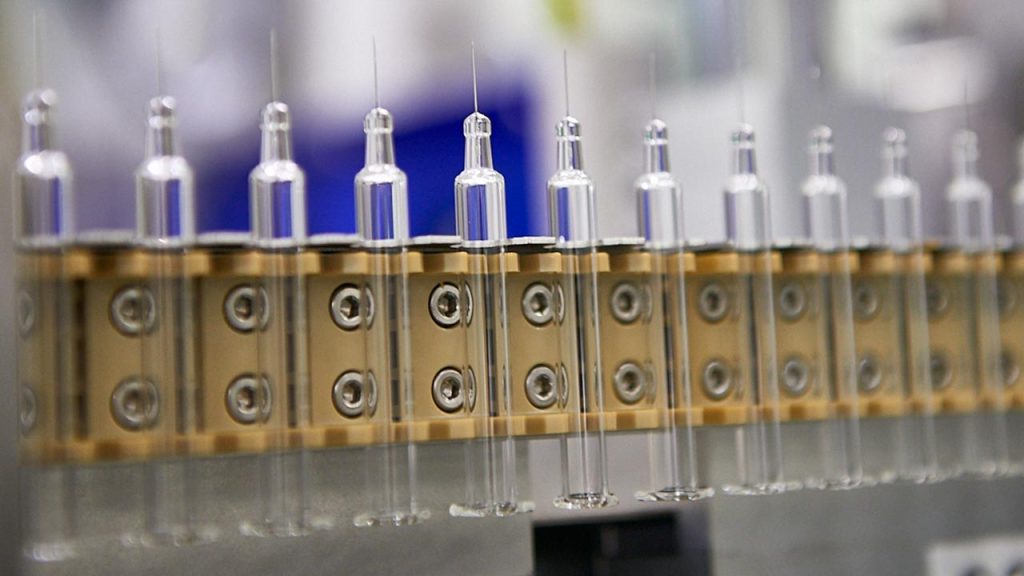
Outcome
Thanks to its vast experience in quality management, particularly in the medical devices sector, Expleo offered its expertise in complex processes and contributed to several parallel transfer projects (around ten transfer projects between 6 and 24 months).
While around twenty transfer projects are completed or underway, the client considers itself “very satisfied” with the quality of the deliverables, the respect of delivery deadlines, the technical expertise and Expleo’s ability to provide solutions.
Creating, as a team, the best solutions to meet our customer’s needs, but also of its factories, its own customers – the pharma labs, and ultimately, of the patients, was a great challenge!
Mailys Fraslin, Project Quality Leader, Expleo.
Manufacturing & Supply Chain